Research Article |
|
Corresponding author: Aylin Ulman ( aylinulman@gmail.com ) Academic editor: Charles Martin
© 2023 Aylin Ulman, Hasan Deniz Akbora, Ozgur Çanak, Elaine Chu, Burak Ali Çiçek, Hasan Ersönmez, Sinan Mavruk, Caner Enver Özyurt, Taner Yildiz, Amy Liu, Nazli Demirel, Daniel Pauly.
This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation:
Ulman A, Akbora HD, Çanak O, Chu E, Çiçek BA, Ersönmez H, Mavruk S, Özyurt CE, Yildiz T, Liu A, Demirel N, Pauly D (2023) A biological and ecological study of the invasive pufferfish Torquigener hypselogeneion (Bleeker 1852) [conspecific Torquigener flavimaculosus Hardy & Randall, 1983] in the Eastern Mediterranean. Aquatic Invasions 18(1): 59-81. https://doi.org/10.3391/ai.2023.18.1.103438
|
Abstract
The highly toxic orange-spotted toadfish Torquigener hypselogeneion (Bleeker 1852) [conspecific Torquigener flavimaculosus Hardy & Randall, 1983] is now a very common invasive fish in the Eastern Mediterranean. Its small size, well under 20 cm, may have concealed the danger it represents, and little is known about its biology or ecology. Here, the spawning seasons, gonado- and hepato-somatic index and condition factors of T. hypselogeneion from 3 locations of the Eastern Mediterranean are presented, based on a total of 1360 individuals sampled, i.e., 216 from Finike, 817 from Fethiye (both Turkey), and 327 from Cyprus. Our results show that T. hypselogeneion is a carnivorous species that forages on sandy bottoms, with a preference for small invertebrates, especially the small invasive gastropod Cerithium scabridum, crustaceans (hermit crabs, other crabs and barnacles), and sea urchins; however, at least in some localities, they appear to forgo eating during their peak reproductive period. The parameters of the von Bertalanffy Growth Function for T. hypselogeneion in the Eastern Mediterranean were: asymptotic length = 17.4 cm (total length; TL) and K = 0.96 year-1, implying a longevity of about 4 years, while the mean length at first maturity was about 10 cm (TL) for both sexes. An average-sized adult female (13 cm TL, 45.7 g live weight) was found to contain 1,250 eggs per gram body weight. Based on its high invasiveness and negative impacts to ecology of the Eastern Mediterranean and the human health, we suggest that T. hypselogeneion should be listed as a priority invasive species and that its population closely monitored within the Mediterranean Sea.
Key words
Invasive Alien Species (IAS), diet, growth, reproduction, spawning season, Tetraodontidae
Introduction
As we transition into a time of declining global marine ecosystem health, increasingly affected by human-induced changes (
The Suez Canal is responsible for most alien species records in the Eastern Mediterranean (
Alien marine species not targeted by fisheries are studied much less than their targeted counterparts, but each alien species needs to be assessed to understand their impacts on the biodiversity, ecosystem function, ecosystem services, as well as human health and socio-economic wellbeing, before managers can understand if a response is required and possible (
There are nearly 200 different species of pufferfishes around the world (
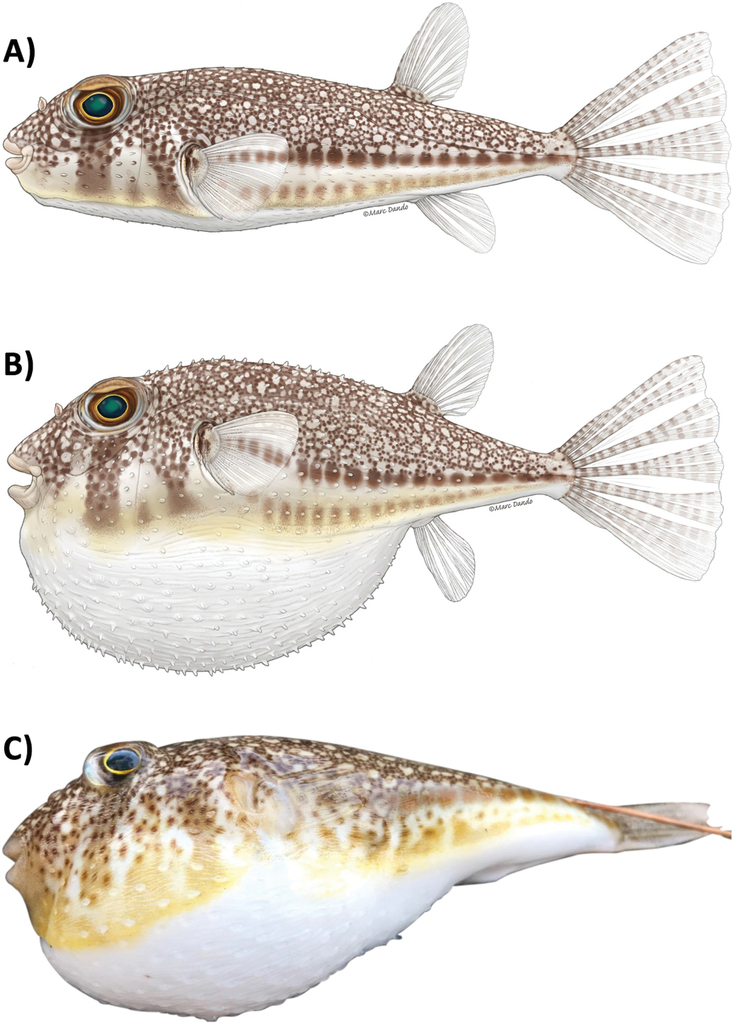
The orange-spotted toadfish Torquigener hypselogeneion (Bleeker 1852) [conspecific Torquigener flavimaculosus Hardy & Randall, 1983], Family Tetraodontidae. A: Lateral view, normal shape; B: The same fish, puffed (both illustrations by Marc Dando). C: Photo of a specimen from Fethiye, Turkey (June 2021; photo by A. Ulman).
Since then, T. hypselogeneion has also spread to Greece, Syria, Egypt, Cyprus and Libya (Figure
Distribution and years of the first records of orange-spotted toadfish Torquigener hypselogeneion (conspecific yellowspotted puffer Torquigener flavimaculosus) in the Mediterranean (1987–2017: red dots), and locations of study sites (2017–2021; yellow dots). The coordinates for the points are provided in Suppl. material
This orange-spotted toadfish T. hypselogeneion may be seen as ‘cute’, with bright emerald eyes lined with gold. It is a demersal species that generally hovers just a few centimeters above the seafloor at depths from 3 to 75 m (
T. hypselogeneion contains an extremely high content of tetradotoxin (TTX), a poison which can be fatal to humans. Pufferfish are considered unsafe for human consumption when they contain over 2 mg·kg-1 (
According to the Aquatic Species Invasiveness Screening Kit (AS-ISK) of five pufferfish species from the Muğla province region in Turkey, T. hypselogeneion scored 31, with species needing to pass the threshold score of 18.5 to be classified as invasive (
Most Mediterranean prior research relates to either new records (
Material and methods
The identification of species was performed based on identification keys provided by
Samples were collected from Finike (36.295 N; 30.141 E), Antalya Province, Turkey by
Locations in Turkey (T) and Cyprus (C) and months/year of sampling specimens of Torquigener hypselogeneion for studies of their Gonadosomatic Index (GSI), Hepato-Somatic Index (HSI), Condition Factor (CF), Stomach Fullness (SF), and Fecundity (F).
| Locality | n | Sampling dates | GSI | HSI | CF | SF | F |
|---|---|---|---|---|---|---|---|
| Fethiyea) (T) | 815 | 3/2020-8/2021 | X | X | X | X | X |
| Finike (T) | 216 | 3/2017-2/2018 | X | X | X | – | – |
| Gazimağusa (C) | 326 | 5/2020-10/2021b | X | X | X | X | – |
Samples of T. hypselogeneion were purchased from small-scale commercial fishers using gill and trammel nets, hooks and lines in Fethiye (Turkey) and Gazimağusa (Cyprus). The price given was 5 Turkish Lira (TRY) for each fish (≈ 0.37 USD). In Finike, fishers were paid a total of 500 TL monthly (≈ 33.5 USD) to collect pufferfish using nets and some samples were also collected personally by the researchers by hook and line. Permission to collect pufferfish from specified fishers for scientific research purposes was granted from the Turkish Ministry of Agriculture and Forestry and General Directorate of Water Products. A total of 1360 pufferfish were sampled from this study, 216 from Finike, 817 from Fethiye (both Turkey), and 327 from Cyprus.
The total length (TL) and live (wet) weight (W) of all fish were measured to the nearest 0.1 cm and the nearest 0.1 g, respectively, while their gonads and livers were weighed to the nearest 0.01 g. The parameter of length-weight relationships (LWRs) of the form W = a·Lb were estimated through re-expression of the LWR equations in linearized form from the 817 Fethiye samples, i.e.,
log(W) = log(a) + b·log(L) (1)
The von Bertalanffy growth function (VBGF), which is commonly used to describe the growth of fish has the form:
Lt = L∞ (1 – e-K(t-t0)) (2)
where Lt is the length at age t, L∞ is the asymptotic length, i.e., the mean length the individuals of a given population would reach if they grew indefinitely, K is the rate, of dimension time-1 (here: year-1) at which L∞ is approached, and t0 is the age at zero length.
Here, a seasonally oscillating version of Equation (2) was used to analyse the available data which has the form:
Lt = L∞{1 – exp – [K(t – t0)+S(t) – S(t0)]} (3)
where S(t) = (CK/2π)·sin(2π(t - ts)), S(t0) = (CK/2π)·sin(2π(t0 - ts)), and L∞, K and t0 are defined as previously, but which has two additional parameters: C and ts. Of these, the former is easiest to visualize, as it expresses the amplitude of the growth oscillations. When C = 0, Equation (3) simplifies back to Equation (2). When C = 0.5, the seasonal growth oscillations are such that growth rate increases by 50% at the peak of the ‘growth season’ (i.e., in ‘summer’), and, briefly, declines by 50% in ‘winter’. When C = 1, growth increases by 100%, doubling during ‘summer’, and becoming zero in the depth of ‘winter’. The other new parameter, ts expresses the time elapsed between t = 0 and the start of a sinusoid growth oscillation. However, visualization is facilitated if we define ts + 0.5 = WP (‘Winter Point’), which expresses, as a fraction of the year, the period when growth is slowest.
Note that longevity (tmax) can be approximated by tmax ≈ 3/K, and that the mutual compatibility of the growth parameters L∞ and K can be evaluated by the growth performance index
Ø’ = log(K) + 2log(L∞) (4)
which should be roughly similar between populations of the same species and between taxonomically related species of similar shapes (
The parameters L∞, K, WP and C of Equation (3) were estimated through the ELEFAN method, which trace multiple growth curves through length-frequency (L/F) samples arranged in time. Each curve passes though peaks (represented by black, positive histograms, and deemed to represent age classes), and through the trough between peaks (represented by white, negative histograms). Peaks and troughs are identified by a simple high-pass filter, i.e., a running average which leads to definition of peaks as those parts of a length-frequency distribution that are above the corresponding running average and conversely for the troughs separating peaks. From a multiplicity of growth curves, each with a different set of growth parameters, the one is retained (along with the values of L∞, K, WP and C which define it) which has the highest score in linking the peaks of L/F distributions, whose ‘point’ values are positive, while avoiding troughs, whose point values are negative (
Only Fethiye samples (Table
The maturity stages of female samples were assigned to one of six stages based on macroscopic examination: immature, developing, developed, spawning capable/running, spent and resting (
Also, the ratio LmaxD/LmD, was computed, where Lmax is the maximum reported length in a population, Lm is a defined in the above paragraph and D = b(1 – d), with b being the exponent of a LWR, (and here set at 3; see below), and d is the exponent of a relationship between the gill surface area (S) and body weight (W) of the form S ∝Wd, with d set at 0.75, as befit a small fish (
For the spawning strategy investigation, we used the peak reproductive period based on increased mean GSI levels measured from the samples for the fecundity and histological sectioning which showed one distinct annual spawning season. Therefore, ovary samples were collected in April and May 2021. The oocyte size–frequency method was used for females with migratory nucleus or early hydrated oocytes to assess their fecundity; here, following
Histological analyses were performed on 90 ovaries. Tissues were removed from the center of each ovary, fixed in 10% formalin solution, dehydrated in an increasingly strong series of ethanol solutions, then embedded in paraffin. Tissue sections of 5 µm, sliced with a microtome were stained with Mayer’s hematoxylin and eosin, then mounted on a slide, then examined with an Olympus BX51 light microscope equipped with an Olympus DP72 digital camera (
Stomach fullness was calculated on a subset of samples which included 465 fish sampled off Fethiye from December 2020 to May 2021 and 327 fish sampled off Cyprus from May 2020 to October 2021 (except for August and September 2020 due to COVID restrictions, compensated for by data for August and September 2021). Stomach fullness was measured using a 4-step scale with the first indicating an essentially empty stomach, the second stomachs that are less than half full, the third stomachs that are more than half full, and the fourth consisting of full stomachs. Next, a visual identification of items in the stomachs of 428 fish from Fethiye was performed using a Zeiss stereoscope; these items were identified to the lowest possible taxa, and then grouped as crustaceans, molluscs, gastropods, bivalves, echinoderms, fish, polychaetes, eggs, seagrass and sand. The number of items were counted in each stomach, if items were partially digested and could still be identified to species level or family or genus level, they were used, if none of those could be determined, then the sample was excluded from the results. If the specimen was identifiable, the following references were used to identify the species:
A Wilcoxon Rank Sum test was used to determine if stomach fullness outside of the spawning season was different from that within the spawning season in Turkey (April-May, 2021 for Fethiye) and Cyprus (April-July 2020). A pairwise Permutational multivariate analysis of variance (PERMANOVA) was used to test whether the differences between sampling locations were statistically significant. For this, a matrix was prepared in the PRIMER software program using total length in cm (TL), weight in g (W), gonad weight in g (Wg), and liver weight in g (Wl) by assigning the location as a fixed factor (
Results
The number, sex and M/F ratio of Torquigener hypselogeneion used in this study are provided in Table
| Location | Finike | Fethiye | Cyprus |
|---|---|---|---|
| Juvenile | 6 | 115 | 8 |
| Female | 109 | 505 | 155 |
| Male | 101 | 197 | 164 |
| M / F | 0.93 : 1 | 0.39 : 1 | 1.06 : 1 |
Illustrating the length-weight relationship (LWR, of the form W = a·Lb) of Torquigener hypselogeneion, based on 817 individuals (sexes combined) sampled near Fethiye, with a = 0.0165 and b = 3.0471 and r2 = 0.907; see also Table
Torquigener hypselogeneion individual GSI results and record details from this study.
| Locality | Date | Sex | TL (cm) | TW (g) | GW (g) | GSI % |
|---|---|---|---|---|---|---|
| Fethiye | May.20 | F | 12.5 | 41.1 | 11.3 | 37.9 |
| Cyprus | Jun.20 | F | 9.5 | 22.4 | 6.68 | 42.5 |
Length-weight relationship parameters for males, females and both sexes pooled for T. hypselogeneion based on Fethiye samples.
| N | a | b | r2 | 95% Confidence Interval of b | |
|---|---|---|---|---|---|
| Male | 197 | 0.0407 | 2.6543 | 0.834 | 2.487–2.821 |
| Female | 505 | 0.0153 | 3.0869 | 0.854 | 2.975–3.199 |
| Pooled | 817 | 0.0165 | 3.0471 | 0.907 | 2.938–3.115 |
Figure
ELEFAN analyses of the length-frequency data of Torquigener hypselogeneion in Suppl. material
Figure
Length at first maturity (Lm) of Torquigener hypselogeneion collected from Fethiye in 2020 and 2021, with the data points fitted logistic curves whose 95% confidence intervals (dotted lines) are also shown, along with the lengths at which the probability of being mature is 0.5. A: Female, with Lm = 9.8 cm. B: Males, with Lm = 10.0 cm. The difference between these two estimates of mean length at first maturity is not significant and Lm ≈ 10.0 cm (TL) for both sexes.
Some specimens had very high GSI values. Thus, from Fethiye, there were ten fish with GSI ranging from 30–38%. From Cyprus, one specimen had a GSI of 42% (Table
The condition factor gradually declined after the onset of the spawning seasons in Fethiye, but Finike samples showed only a very slight dip. In Northern Cyprus, the condition factor peaked in June right after the start of the peak spawning season.
According to the PERMANOVA results: total length, total weight, gonad weight, and liver weight were significantly different by means of locations (Table
The pairwise Permutational multivariate analysis of variance (Permanova) results compared for the study areas.
| Pairs | Degrees of freedom | p value | Pseudo-F statistics |
| Fethiye-Cyprus | 897 | 0.001 | 87.240 |
| Fethiye-Finike | 791 | 0.001 | 52.410 |
| Cyprus-Finike | 368 | 0.008 | 26.963 |
The first and second principal component axes (PC1 and PC2) explained about 76% and 14% of the variation among the length and weight variables, respectively. Positive regressions for all four variables (length and body weight, gonad liver weight) were correlated with PC1, whereas PC2 negatively correlated with gonad weight and strongly correlated with length (Table
Variance in 4 traits of Torquigener hypselogeneion sampled at 3 sites explained by the first two axes of a Principle Component Analysis (PCA).
| Variable | PC1 (75.7%) | PC2 (13.8%) |
| Total length | 0.501 | 0.584 |
| Body weight | 0.552 | 0.234 |
| Liver weight | 0.492 | -0.165 |
| Gonad weight | 0.449 | -0.760 |
The PCA result shows that adult fish from Finike (N = 210) and Cyprus (N = 318) do not appear to differ, whereas the fish sampled in Fethiye (N = 678) showed a greater variance along both axes (Figure
The ovaries of T. hypselogeneion appear to be organized into synchronous groups, i.e., they show two distinct sized group of ovaries, but fecundity appears to be determinate. Primary growth (PG) oocytes and vitellogenic oocytes (Vit) were clearly recognized during the spawning period (Figure
Stages of oocyte development in Torquigener hypselogeneion. Histological sections show A: primary growth oocytes (PG), vitellogenic oocytes (Vit) and atresia (At); B: primary growth oocytes (PG) and hydrated oocytes (H); and C: vitellogenic oocytes (Vit), post ovulatory follicles (POF) and atresia (At). Scale bars 800 μm (A, B); 400 μm (C).
The fecundity study was conducted on females with ripe ovaries ranging from 11.8 to 15.8 cm in total length and 31.6 and 78.7 g in weight during the peak spawning season (mostly from late April, 2021). Late development phase of oocytes (late vitellogenesis) was examined under the microscope. Fecundity was found to be between 448 to 3,165 eggs per gram body weight and the fecundity of an average-sized female (13 cm TL and 48 g in W) T. hypselogeneion was found to be 1,250 eggs per gram body weight. No relationship could be established between fecundity and fish size.
Table
Overall stomach fullness (SF) in %, as evaluated on a 4-point scale, i.e., ‘Empty’ (0), ‘Up to ½ full’ (0< - <0.5), ‘More than ½ full’ (≥ ½ - <1) and ‘Full’ (1), of specimens of Torquigener hypselogeneion from Fethiye and Cyprus.
| Stomach Fullness | N | 0 | 0< - <0.5 | ≥ 1/2 - <1 | 1 |
|---|---|---|---|---|---|
| Fethiye | 465 | 21 | 36 | 29 | 14 |
| Cyprus | 327 | 38 | 25 | 18 | 19 |
In Fethiye samples, small gastropods made up much of the diet, with the invasive Cerithium scabridum Philippi, 1848 (10–25 mm in length), along with similar-sized gastropods such as Bittium reticulatum (da Costa, 1778), Phorchus turbinatus (Born, 1778) being found in 46% of specimens, followed by crustaceans with 32%, which mainly included hermit crabs using gastropod shells as their shelter, crabs (mainly juvenile Calappa granulata (Linnaeus, 1767)), barnacles (with entire shells), and squat lobsters (Galathea squamifera Leach, 1814). The third most frequent prey group was sea urchins. Here, both native species were identified (Arbacia lixula (Linnaeus, 1758) and Paracentrotus lividus (Lamarck, 1816). Bivalves were found in 7% of the stomachs and included juvenile forms of Chamelea gallina (Linnaeus, 1758), Clausinella fasciata (da Costa, 1758), Arculata senhousia (Benson, 1842). Other items in 1–3% of stomachs were Polititapes aureus (Gmelin, 1791), seagrass, small fish and fish eggs, polychaetes and cephalopods, via their long-lasting ink. In Cyprus, the stomach contents consisted mainly of crustaceans, with crabs contributing 44%, hermit crabs 23% and barnacles 17%, followed by cephalopod ink 9%, and gastropods and fish contributing the rest.
Discussion
This study provides new information on its biological characteristics such as growth, spawning season, reproduction and diet for T. hypselogeneion, the tiny but deadly invasive pufferfish in the Mediterranean. In comparing three different populations, features are emphasized which pertain to the Fethiye specimens, where this species apparently first established itself in the Mediterranean (
The maximum length sampled in Fethiye was 16.3 cm (TL), very close to the 16.5 cm maximum length reported by
Comparison of the growth performance of Torquigener hypselogeneion with that of other pufferfish species using ᴓ’ = log(K)+2log(L∞).
| Species | L∞ (cm) | K (year-1) | ᴓ’ | Reference |
|---|---|---|---|---|
| Lagocephalus sceleratus ♀&♂ | 82.0 | 0.5 | 3.52 |
|
| 88.7 | 0.27 | 3.43 |
|
|
| 81.1 | 0.26 | 3.23 |
|
|
| Sphoeroides maculatus ♀ | 28.2 | 0.607 | 2.68 |
|
| Sphoeroides maculatus ♂ | 24.5 | 0.620 | 2.57 |
|
| Sphoeroides testudineus ♀&♂ | 30.0 | 0.51 | 2.66 |
|
| T. hypselogeneion ♀&♂ | 17.4 | 0.96 | 2.46 | This study |
| Contusus richei ♀ | 18.9 | 0.326 | 2.07 |
|
| Contusus richei ♂ | 12.5 | 0.362 | 1.77 |
|
A very low male to female ratio (M:F = 0.39:1) was found for all months Fethiye, whereas Finike had a ratio of 0.93:1, and Cyprus had a ratio of 1.06:1. Another recent study from Cyprus reported a very high male to female ratio close to 4:1 (
Torquigener hypselogeneion appears to reach maturity at 1 year, when its LmaxD/LmD ratio is about 1.44, which is well within the 95% confidence interval (1.22–1.53) of the 1.35 threshold value shown to trigger first maturation and spawning in bony fishes (
Of our three sites, the spawning season was longest in Fethiye (lasting from March to August in 2020), then Cyprus (from March to July), while it lasted only two months in Finike (April to May). These three sites are at about the same latitude, but the mean water temperature is slightly higher in the more eastward Cyprus. This somewhat aligns with the results on an extensive study by
An extensive study on GSI values for Mediterranean fish species that analyzed 237 stocks belonging to 81 species reported a Mediterranean mean GSI value of 6.8% (in spawning season only) for both sexes, 8.6% for females and a significantly lower rate of 4.2% for males (
For Fethiye and Finike, as expected, the peak hepato-somatic index (HSI) occurred one month before the peak spawning period, and then a gradual decrease in HSI was observed as energy reserves were used for gonadal development, as found in other studies (
The stark differences in the spawning periods between our three sites is intriguing. Based on GSI values, an extended period was found in Fethiye. However, we performed fecundity studies only in late April, and histological studies in May, i.e., both during peak spawning season. Because there was another slight peak in GSI values during autumn period, total fecundity per annum per female was not estimated. Additionally, we could not evaluate whether this species continued to spawn, or whether the ovaries transform to atretic stages. Future studies should cover both the spawning and non-spawning period to better understand its adaptation mechanisms considering any possible changes to its reproductive strategies, as well as total fecundity of this species for determining annual egg production.
Another intriguing result was that the fecundity of T. hypselogeneion did not vary with size, i.e., small individuals contained as many eggs as large ones. One explanation for this may be found in the morphological adaptations which allow pufferfish to puff, such as their stretchable skin and lack of pleural ribs and pelvis. Possibly, this allows for an extra space for the storage of eggs that may be (between lengths of 10 to 16 cm), independent of body size. This would also explain why, at least in Fethiye, feeding is reduced during the spawning season, which would also allow the abdominal cavity to accommodate more eggs. Oocyte sizes during vitellogenesis are very small (avg. 350 μm in diameter), which allows for a high fecundity. During hydration, egg size may strictly increase (
While its extremely powerful regenerative fused teeth (
In the waters off of Cyprus, the native sea urchin populations of P. lividus started declining around 2010 and collapsed in 2014 (
From in situ observations of T. hypselogeneion in their environments in both Turkey and Cyprus, aggressive and competitive feeding behaviour was noticed on a few occasions in the presence of blood from spearfishing lionfish and dead crabs from cleaning lost fishing nets.
Conclusion
Marine invasive species research is highly interesting in the Eastern Mediterranean, as the marine ecosystems are undergoing a major transition caused by the influx of alien species. Hence some of their adaptations may differ at separate locations based on differences in the ecosystem structure or abiotic variables. The three Torquigener hypselogeneion [conspecific Torquigener flavimaculosus] populations examined here showed some considerable variabilities with respect to sex ratios, spawning periods and diet compositions. Fethiye, where they were first noticed in high abundances in the Mediterranean seems to have favourable conditions for its growth and reproduction, with the highest GSI, and fecundity estimated for the first time. Our results are comparable with those for other small pufferfish; maturity is reached after one year, and longevity is about 4 years; the males become thinner as they grow, while the females maintain the same body shape. Fecundity is high, and largely size independent. The food mainly consists of small benthic animals, either chomped by powerful fused teeth, or likely blown of sandy seafloor be jets of waters, two adaptations providing access to a wide range of resources. Based on its high invasiveness and negative impacts to ecology of the Eastern Mediterranean and the human heath, we suggest that T. hypselogeneion be listed as a priority invasive species and that its population and impacts be closely monitored within the Mediterranean Sea.
Funding declaration
D.P.’s and E.C.’s research is funded by the Sea Around Us, itself funded by a number of philanthropic foundations, notably the Oak, Marisla and Packard Foundations under a variety of grants. The Finike study (C.E.Ö., H.E., and S.M.) research was funded by Çukurova University Scientific Projects Coordination Unit, Project number: FDK-2017-8673. The other authors have no funding to report.
Ethics and permits
This research was carried out under research permissions granted from the Turkish Ministry of Agriculture and Forestry and General Directorate of Water Products under permission #67852565-140.03.03-E 1354602 & #6987137-663.08 to collect pufferfish for scientific research purposes.
Author contributions
AU- Conceptualization; AU, HE, CEO, BAC, HDA- Sample Design & Methodology; AU, BAC, HDA, HE, OC, ND, AL, SM- Investigation; AU, EO, CEO, BAC, HAD- Resources; TY, ND, DP, EC- Software; AU, HE, CEO, SM, BAC, HDA- Validation; AU, ND, CEO, HE, DP, EC- Formal analysis; AU, TY- Data Curation; AU, ND- Writing - Original draft; AU, ND, DP- Writing - Review and Editing; AU, TY, ND, DP, EC, AL-Visualization.
Acknowledgements
We thank Marc Dando for the exceptional scientific illustration. We also thank Rainer Froese for providing papers of interest. We also are indebted to the anonymous reviewers for their valuable time and care they took with improving our article.
References
- Al Mabruk S, Stoilas VO, Kleitou P, Giovos I (2018) The first record of Torquigener flavimaculosus (Tetraodontiformes: Tetraodontidae) from Libya. International Journal of Fisheries and Aquatic Studies 6(4): 449–450.
- Arthington AH, Dulvy NK, Gladstone W, Winfield IJ (2016) Fish conservation in freshwater and marine realms: status, threats and management. Aquatic Conservation: Marine and Freshwater Ecosystems 26: 838–857. https://doi.org/10.1002/aqc.2712
- Ayas D, Gürlek M, Çiftçi N, Doğdu SA, Akbora HD, Moez S, Turan C (2019) Length-weight relationships of pufferfish species (Tetraodontidae Bonaparte, 1832) from Mersin Bay, Northeastern Mediterranean Sea. Proceeding of Next Generation Biometry Workshop and Course. Published by Natural and Engineering Sciences, Iskenderun, Turkey, 18–25.
- Ballesteros E, Llobet T (2015) Marine wildlife of the Mediterranean. Gallocanta, Spain, 144 pp.
- Bane V, Lehane M, Dikshit M, O’Riordan A, Furey A (2014) Tetrodotoxin: Chemistry, toxicity, source, distribution and detection. Toxins 6(2): 693–755. https://doi.org/10.3390/toxins6020693
- Bilecenoğlu M (2003) Kızıldeniz Göçmeni Balon Balığı (Torquigener flavimaculosus Hardy & Randall, 1983), Türkiye kiyilarindan ilk gözlemler. Sualti Dünyasi Dergisi 74: 38–39. [in Turkish]
- Bilecenoğlu M (2005) Observation on the burrowing behaviour of the dwarf blaasop, Torquigener flavimaculosus (Osteichthyes: Tetraodontidae) along the coast of Fethiye, Turkey. Zoology in the Middle East 35: 29–34. https://doi.org/10.1080/09397140.2005.10638100
- Bilecenoglu M, Yokes MB (2022) Torquigener flavimaculosus Hardy & Randall, 1983 (Actinopteri: Tetraodontidae), a junior synonym of Torquigener hypselogeneion (Bleeker, 1852) based on molecular and morphological data. Zoology in the Middle East 68(4): 309–319. https://doi.org/10.1080/09397140.2022.2121082
- Çek-Yalnız S, Turan F, Doğdu S (2017) Maturation and gonad development of yellowspotted puffer Torquigener flavimaculosus (Osteichthyes: Tetraodontidae) from Iskenderun Bay, North-eastern Mediterranean. Natural and Engineering Sciences 2(3): 1–11. https://doi.org/10.28978/nesciences.368991
- Chartosia N, Michailidis N, Constantinou A, Karachle PK (2021) Shedding light on the diet of the Lessepsian yellowspotted puffer Torquigener flavimaculosus Hardy & Randall, 1983 in the Eastern Mediterranean. Acta Adriatica 62(2): 199–208. https://doi.org/10.32582/aa.62.2.7
- Chen Z, Bigman J, Xian W, Liang C, Chu E, Pauly D (2021) The ratio of length at first maturity to maximum length in marine and freshwater fishes. Journal of Fish Biology 101(2): 400–407. https://doi.org/10.1111/jfb.14970
- Çiçek BA (2019) Non-indigenous fish species in north of Cyprus: ecological status and impacts on key habitats. In: Langar H, Ouerghi A (Eds) UNEP/MAP – SPA/RAC, 2019. Proceedings of the 1st Mediterranean Symposium on the Non-Indigenous Species (Antalya, Turkey, 18 January 2019). SPA/ RAC publication, Tunis 2019, 40–44.
- Clarke KR, Gorley RN (2001) Primer v5: User Manual/Tutorial. Primer-E Ltd., Plymouth, 91 pp.
- Clarke KR, Gorley RN (2006) PRIMER v6: User Manual/Tutorial (Plymouth Routines in Multivariate Ecological Research). PRIMER-E, Plymouth.
- Corsini-Foka M, Margıes P, Kondilatos G, Economidis P (2006) Torquigener flavimaculosus Hardy & Randall, 1983 (Pisces: Tetraodontidae) off Rhodes island marine area: a new alien fish in the Hellenic waters. Mediterranean Marine Science 7(2): 73–76. https://doi.org/10.12681/mms.172
- Darling E, Green S, O’Leary J, Côté I (2011) Indo-Pacific lionfish are larger and more abundant on invaded reefs: A comparison of Kenyan and Bahamian lionfish populations. Biological Invasions 13: 2045–2051. https://doi.org/10.1007/s10530-011-0020-0
- Demirel N, Ulman A, Yildiz T, Ertör-Akyazı P (2021) A moving target: Achieving good environmental status and social justice in the case of an alien species, Rapa whelk, in the Black Sea. Marine Policy 132(2): 104687. https://doi.org/10.1016/j.marpol.2021.104687
- Edelist D (2014) New length–weight relationship and Lmax values for fishes from the Southeastern Mediterranean Sea. Journal of Applied Ichthyology 30: 521–526. https://doi.org/10.1111/j.1439-0426.2012.02060.x
- Ergüden D, Ergüden SA, Gürlek M (2015) Length-weight relationships for six fish species in Iskenderun Bay (Eastern Mediterranean Sea coast of Turkey). Journal of Applied Ichthyology 31: 1148–1149. https://doi.org/10.1111/jai.12839
- Ergüden A, Ayas D, Ergüden D (2020) The length-weight relationship and condition factor of yellowspotted puffer Torquigener flavimaculosus Hardy & Randall, 1983 in the Eastern Mediterranean (Yumurtalık Bight, Turkey). Marine Science and Technology Bulletin 9(2): 87–91.
- Ersönmez H (2019) Population parameters and feeding properties of some puffer fish in Finike Bay (Antalya). PhD Thesis, Çukurova University Institute of Natural and Applied Sciences, Çukurova, 154 pp.
- Farrag MSM, El-Haweet AAK, Akel EA, Moustafa MA (2016) Occurrence of puffer fishes (Tetraodontidae) in the eastern Mediterranean, Egyptian coast - filling in the gap. Bioinvasions Records 5(1): 47–54. https://doi.org/10.3391/bir.2016.5.1.09
- Fernandez de Puelles ML, Gras D, Hernandez-Leon S (2003) Annual cycle of zooplankton biomass, abundance and species composition in the neritic area of the Balearic Sea, Western Mediterranean. PSZN Marine Ecology 24: 123–139. https://doi.org/10.1046/j.1439-0485.2003.03816.x
- Filiz H, Yapıcı S, Bilge S (2017) The Factors Increasing of Invasiveness Potential of Five Pufferfishes in the Eastern Mediterranean, Turkey. Natural and Engineering Sciences 2(3): 22–30. https://doi.org/10.28978/nesciences.369004
- Froese R, Pauly D [Eds] (2022) FishBase. World Wide Web electronic publication. www.fishbase.org [Accessed on 01.09.2022]
- Galil B, Marchini A, Occhipinti-Ambrogi A, Ojaveer H (2017) The enlargement of the Suez Canal – Erythraean introductions and management challenges. Management of Biological Invasions 8: 47–54. https://doi.org/10.3391/mbi.2017.8.2.02
- Gaudy R, Champalbert G (1998) Space and time variations in zooplankton distribution south of Marseilles. Oceanologica Acta 21: 793–802. https://doi.org/10.1016/S0399-1784(99)80007-3
- Gayanilo FC, Sparre P, Pauly D (2005) FAO-ICLARM Stock Assessment Tools II (FiSAT II). Revised Version User’s Guide. FAO Computerized Information Series (Fisheries), No. 8. FAO, Rome, 168 pp. [Distributed with one CD-ROM]
- Golani D (1987) The Red Sea pufferfish, Torquigener flavimaculosus Hardy and Randall 1983, a new Suez Canal migrant to the eastern Mediterranean. (Pisces: Tetraodontidae). Senckenbergiana Maritima 19(5/6): 339–343.
- Golani D, Azzurro E, Dulčić J, Massutti E, Orsi-Relini L, Briand F (2021) CIESM Atlas of exotic fishes in the Mediterranean Sea. CIESM Publishers, Paris, Monaco, June 2021, 365 pp.
- Hardy GS (1983) Revision of Australian species of Torquigener Whitley (Tetraodontiformes: Tetraodontidae), and two new generic names for Australian puffer fishes. Journal of the Royal Society of New Zealand 13: 1–48. https://doi.org/10.1080/03036758.1983.10415335
- Hardy GS (1984) Redescription of the pufferfish Torquigener brevipinnis (Tetraodontidae), with description of a new species of Torquigener from Indonesia. Pacific Science 38(2): 127–133.
- Hardy GS, Randall JE (1983) Description of a new species of pufferfish (Tetraodontiformes: Tetraodontidae) from the Red Sea and adjacent waters. Israel Journal of Zoology 32(1): 13–20.
- Hotelling H (1933) Analysis of a complex of statistical variables into principal components. Journal of Educational Psychology 24(6): 417–441. https://doi.org/10.1037/h0071325
- Laroche JL, Davis J (1973) Age, growth and reproduction of the northern puffer, Sphoeroides maculatus. U.S. Fishery Bulletin 71: 955–963.
- Katikou P (2019) Public health risks associated with tetrodotoxin and its analogues in European waters: Recent advances after the EFSA scientific opinion. Toxins 11(5): 240. https://doi.org/10.3390/toxins11050240
- Katsanevakis S, Wallentinus I, Zenetos A, Leppäkoski E, Çinar M, Oztürk B, Grabowski M, Golani D, Cardoso A (2014) Impacts of invasive alien marine species on ecosystem services and biodiversity: a pan-European review. Aquatic Invasions 9: 391–423. https://doi.org/10.3391/ai.2014.9.4.01
- Kebapçioğlu T, Beğburs C (2017) Pufferfish species as discards in bottom trawl fisheries of Gulf of Antalya and Finike bay, turkey. Supplement of abstracts, Abstracts Book of International Symposium on Pufferfish, 13–14 October 2017, Bodrum, Turkey, 36 pp.
- Kosker AR, Özogul F, Durmus M, Ucar Y, Ayas D. Šimat V, Özogul Y (2018) First report on TTX levels of the yellow spotted pufferfish (Torquigener flavimaculosus) in the Mediterranean Sea. Toxicon 148: 101–106. https://doi.org/10.1016/j.toxicon.2018.04.018
- Leuzinger S, Rewald B (2021) The Who or the How? Species vs. Ecosystem Function Priorities in Conservation Ecology. Frontiers in Plant Science 12: 758413. https://doi.org/10.3389/fpls.2021.758413
- Martinou A, Pescott O, Michailidis N, Zenetos A, Jenna Wong L, Pagad S (2018) Global Register of Introduced and Invasive Species - Cyprus. Invasive Species Specialist Group ISSG. Checklist dataset. https://doi.org/10.15468/uryl57
- Mavruk S, Saygu İ, Bengil F (2020) Fishers’ responses towards the banning white grouper fishery in Turkey. Journal of Wildlife and Biodiversity 4(4): 50–57. https://doi.org/10.22120/jwb.2020.136593.1187
- Michailidis N (2010) Study on the lessepsian migrant Lagocephalus sceleratus in Cyprus. EastMed Technical Documents. In: Report of the technical meeting on the lessepsian migration and its impact on eastern Mediterranean fishery. Athens, FAO, 74–87.
- Murua H, Kraus G, Saborido-Rey F, Witthames PR, Thorsen A, Junquera S (2003) Procedures to estimate fecundity of marine fish reproduction of Mediterranean horse mackerel species in relation to their reproductive strategy. Journal of Northwest Atlantic Fishery Science 33: 33–54. https://doi.org/10.2960/J.v33.a3
- Mutlu E, De Meo I, Miglietta C (2021) Spatio-temporal distribution of pufferfish (Tetraodontidae) along the Turkish coast of the Mediterranean Sea. Mediterranean Marine Science 22(1): 1–19. https://doi.org/10.12681/mms.23481
- Mutlu E, De Meo I, Miglietta C, Deval CM (2023) Keystone porgy species (Sparidae) overcome the alien Randall’s threadfin bream (Nemipterus randalli) for catch balance in space on an oligotrophic Levant Shelf or vice versa? COMU Journal of Marine Sciences and Fisheries 5(2): 119–142. https://doi.org/10.46384/jmsf.1159667
- Nissling A, Nyberg S, Petereit C (2017) Egg buoyancy of flounder, Platichthys flesus, in the Baltic Sea—adaptation to salinity and implications for egg survival. Fisheries Research 191: 179–189. https://doi.org/10.1016/j.fishres.2017.02.020
- Noguchi T, Ebesu JSM (2001) Puffer poisoning: Epidemiology and treatment. Journal of Toxicology Toxin Reviews 20: 1–10. https://doi.org/10.1081/TXR-100103080
- Pauly D (1984) A mechanism for the juvenile-to-adult transition in fishes. ICES Journal of Marine Science 41: 280–284. https://doi.org/10.1093/icesjms/41.3.280
- Pauly D (1991) Growth of the checkered puffer Sphoeroides testudineus: postscript to papers by Targett and Pauly & Ingles. Fishbyte, Newsletter of the Network of Tropical Fisheries Scientists 9(1): 19–22.
- Pauly D (1998) Beyond our original horizons: the tropicalization of Beverton and Holt. Reviews in Fish Biology and Fisheries 8(3): 307–334. https://doi.org/10.1023/A:1008863215253
- Pauly D (2021a) The Gill-Oxygen Limitation Theory (GOLT) and its critics. Science Advances 7(2): eabc6050. https://doi.org/10.1126/sciadv.abc6050
- Pauly D (2021b) Why do fish reach first maturity when they do? Journal of Fish Biology 101(2): 333–341. https://doi.org/10.1111/jfb.14902
- R Core Team (2021) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
- Ramadan MA, El-Halfawy MM (2019) Reproductive biology of the yellowspotted puffer Torquigener flavimaculosus (Osteichthyes: Tetraodontidae) from Gulf of Suez, Egypt. Egyptian Journal of Aquatic Biology & Fisheries 23(3): 503–511. https://doi.org/10.21608/ejabf.2019.51022
- Roberts RJ, Smail DA, Munro ES (2012) Laboratory methods. In: Roberts R (Ed.) Fish Pathology, 4th ed. , Wiley & Blackwell, 439–481. https://doi.org/10.1002/9781118222942.ch12
- Sabour W, Saad A, Jawad L (2015) First record of the yellow spotted puffer Torquigener flavimaculosus Hardy & Randall, 1983 (Osteichthyes:Tetraodontidae) from the Mediterranean Sea Coasts of Syria. Thalassia Salentina 36: 29–34.
- Sabrah MM, El-Ganainy AA, Zaky MA (2006) Biology and toxicity of the puffer fish Lagocephalus sceleratus (Gmelin, 1789) from the Gulf of Suez. Egyptian Journal of Aquatic Biology and Fisheries 32: 283–297.
- Sala E, Kizilkaya Z, Yildirim D, Ballesteros E (2011) Alien Marine Fishes Deplete Algal Biomass in the Eastern Mediterranean. PLoS ONE 6(2): e17356. https://doi.org/10.1371/journal.pone.0017356
- Salimi P, Creed JC, Esch M, Fenner D, Jaafar Z, Levesque J, Montgomery A, Salimi M, Edard J, Raj K, Sweet M (2021) A review of the diversity and impact of invasive non-native species in tropical marine ecosystems. Marine Biodiversity Records 14: 11. https://doi.org/10.1186/s41200-021-00206-8
- Steffen W (2021) Introducing the Anthropocene: The human epoch. Ambio 50: 1784–1787. https://doi.org/10.1007/s13280-020-01489-4
- Steffen W, Persson Å, Deutsch L, Zalasiewicz J, Williams M, Richardson K, Crumley C, Crutzen P, Folke C, Gordon L, Molina M, Ramanathan V, Rockström J, Scheffer M, Schellnhuber HJ, Svedin U (2011) The Anthropocene: From global change to planetary stewardship. Ambio 40: 739–761. https://doi.org/10.1007/s13280-011-0185-x
- Thiery AP, Shono T, Kurokawa D, Britz R, Johanson Z, Fraser GJ (2017) Pufferfish beak regeneration. Proceedings of the National Academy of Sciences 114(22): E4425–E4434. https://doi.org/10.1073/pnas.1702909114
- Tsikliras A, Antonopoulou E, Stergiou K (2010) Spawning period of Mediterranean marine fishes. Reviews in Fish Biology and Fisheries 20: 499–538. https://doi.org/10.1007/s11160-010-9158-6
- Turna İ, Ertan O, Cormaci M, Furnari G (2002) Seasonal variations in the biomass of macro-algal communities from the Gulf of Antalya (north-eastern Mediterranean). Turkish Journal of Botany 26(1): 4. https://journals.tubitak.gov.tr/botany/vol26/iss1/4
- Ulman A, Ferrario J, Forcada A, Arvantidis C, Occhipinti-Ambrogi A, Marchini A (2019) A hitchhiker’s guide to alien species settlement in Mediterranean marinas. Journal of Environmental Management 241: 328–339. https://doi.org/10.1016/j.jenvman.2019.04.011
- Ulman A, Yildiz T, Demirel N, Canak O, Yemişken E, Pauly D (2021) The biology of the invasive silver-cheeked toadfish (Lagocephalus sceleratus), with emphasis on the Easter Mediterranean. Neobiota 68(3): 145–175. https://doi.org/10.3897/neobiota.68.71767
- Ulman A, Kalogirou S, Pauly D (2022) The dynamics of maximum lengths in the invasive silver-cheeked toadfish (Lagocephalus sceleratus) in the Eastern Mediterranean Sea. Journal of Marine Science & Engineering 10: 387. https://doi.org/10.3390/jmse10030387
- van Damme C (2010) Determinate and indeterminate fecundity types in marine fish: a conceptual model. Lecture notes. IMARES Vis, Wageningen University, 12 pp.
- Wainwright PC, Turingan RG (1997) Evolution of pufferfish inflation behavior. Evolution 51(2): 506–518. https://doi.org/10.1111/j.1558-5646.1997.tb02438.x
- Wirtz P, Debelius H (2003) Mediterranean and Atlantic Invertebrate Guide. Conchbooks, Hackenheim, Germany, 305 pp.
- Yeruham E, Rilov G, Shpigel M, Abelson A (2015) Collapse of the echinoid Paracentrotus lividus populations in the Eastern Mediterranean—result of climate change? Scientific Reports (5): 13479. https://doi.org/10.1038/srep13479
- Zenetos A, Vassilopoulou V, Salomidi M, Poursanidis D (2007) Additions to the marine alien fauna of Greek waters (2007 update). Marine Biodiversity Records 1: E91. https://doi.org/10.1017/S1755267207009281
- Zenetos A, Albano PG, López Garcia E, Stern N, Tsiamis K, Galanidi M (2022) Established non-indigenous species increased by 40% in 11 years in the Mediterranean Sea. Mediterranean Marine Science 23(1): 196–212. https://doi.org/10.12681/mms.29106
Supplementary materials
Length-frequency data of Torquigener hypselogeneion collected in Finike, Turkey from March 2017 to February 2018
Data type: table (docx. file)
Length-frequency data of Torquigener hypselogeneion collected in Fethiye from March 2020 to August 2021
Data type: table (docx. file)
Length-frequency data of Torquigener hypselogeneion collected in Cyprus from May 2020 to April 2021 with additional data for August and September 2021
Data type: table (docx. file)
Georeferenced coordinates for study sites and first records provided in Fig.
Data type: table (docx. file)